Polyesters
Properties
Polyesters are one of the most important classes of thermoplastic polymers.
They can be formed from the reaction of a diacid or acid anhydride and a diol with the
elimination of water, or by ring-opening polymerization of cyclic (di-)esters.
According to the composition of their main chain, polyesters are classified as aliphatic, semi-aromatic
and
aromatic (see table below). Aromatic reactants improve the hardness, rigidity, and heat resistance, whereas
aliphatic acids and diols increase the flexibility, lower the melting or softening point and improve the
processability.
Common aliphatic diols are ethylene glycol,
1,4-butanediol, and 1,3-propanediol. They are often reacted with
aromatic diacids, such as terephthalic acid, phthalic acid, phthalic anhydride and naphthalene dicarboxylic acid.
Glycerol and unsaturated acids (anhydrides) like maleic anhydride, are sometimes added to crosslink the polyesters.
In the case of unsaturated acids (anhydrides), crosslinking is achieved in a subsequent free radical chain polymerization.
Double bonds in the backbone of the polyesters also improve the resistance to softening and deformation at elevated
temperatures.
A large number of polyesters exist due to the numerous possible combinations of dialcohols and diacids. However, only a small number have gained commercial significance. The most important commercial polyesters are poly(ethylene terephthalate) (PET) and poly(butylene terephthalate) (PBT). Both are semi-aromatic and are either amorphous when solidified by rapid cooling or semi-crystalline when solidified slowly. Both polyesters can be easily molded or thermoformed and have many attractive properties, like high strength and toughness, good abrasion and heat resistance, low creep at elevated temperatures, good chemical resistance and excellent dimensional stability, particularly when glass-fiber reinforced.
Another important polyester is polyethylene naphthalate (PEN). Compared with PET, it has lower oxygen permeability, improved hydrolytic stability, higher tensile strength and service temperature, and reduced elongation and shrinkage due to the higher Tg (120°C vs 75°C). PEN usually surpasses PET in top end demanding applications and is often a good and less expensive alternative to polyimides.
Completely aliphatic polyesters, made from aliphatic diacid and aliphatic diol components, are produced on a much smaller scale. They have low melting or glass transition temperatures and poor hydrolytic stability. They are mainly used as low-molecular-weight plasticizers and as prepolymer reactants in the synthesis of polyurethanes. A few other aliphatic polyesters are known for their biocompatibility and biodegradability, and their capability to be blended with various other commercial polymers. Among these lactones like ε-caprolactone have found commercial use which can be ring-opening polymerized with various types of initiators. Polycaprolactone (PLC) has good water, oil, and solvent resistance. This polymer is often blended with other resins to improve their processing and end use properties. It can also be blended with starch to lower cost and to increase the biodegradability. PLC resin blends are also used as a feedstock for injection-molding of disposable articles like drinking cups and food containers.However, the market share of biodegradable polymers is rather small but is expected to grow.1
Fully aromatic polyesters have found very few commercial applications. Due to their high crystallinity, they are difficult to process. They have high softening points (480 - 630 K), good dielectric strength, excellent mechanical properties, and good heat resistance. For example, the aromatic polyester produced by the polycondensation of 4-hydroxybenzoic acid and 6-hydroxynaphthalene-2-carboxylic acid (Vectran LCP) is on a pound-for-pound basis five times stronger than steel. Other important aromatic polyesters are polyarylates (PAR). They are amorphous thermoplastics produced from terephtalic acid or phthalic acid, and bisphenols. They are highly crystalline polymers with similar high heat and chemical resistance.
| Polyester Subclass | Manufacturing Methods | Examples |
| Aliphatic Polyesters | Polycondensation, Ring-opening polymerization | Polycaprolactone (PCL) Polylactic acid (PLA) Polyhydroxybutyrate (PHB) Polyglycolic acid (PGA) Polyethylene adipate (PEA) |
| Semi-Aromatic Polyesters | Polycondensation | Polyethylene terephthalate (PET) Polybutylene terephthalate (PBT) Polyethylene naphthalate (PEN) Polytrimethylene terephthalate (PTT) |
| Aromatic Polyesters (Polyarylates) | Polycondensation | Polyester of 4-hydroxybenzoic acid and 6-hydroxynaphthalene-2-carboxylic acid (LCP) Polyester of Bisphenol A and phthalic acid (PAR) |
COMMERCIAL POLYESTERS
Major manufacturers of unfilled and filled semi-aromatic polyesters are SABIC, DuPont, Invista, BASF and Celanese. The most important resins are PET and PBT.
A large number of commercial grades are available. Many are reinforced with up to 50 percent glass. Other important semi-aromatic polesters are
polytrimethylene terephthalate (PTT) and polyethylene naphthalate (PEN).
Major manufacturers of aromatic polyesters are Kuraray ( Vectran®),
Celanese ( Vectra®),
and DuPont ( Zenite LCP®).
| Polyester | Structure of Repeat Unit | Trade Name |
| Polyethylene terephthalate (PET,PETE) |

| Mylar®, Rynite®, Impet®, |
| Polybutylene terephthalate (PBT) |
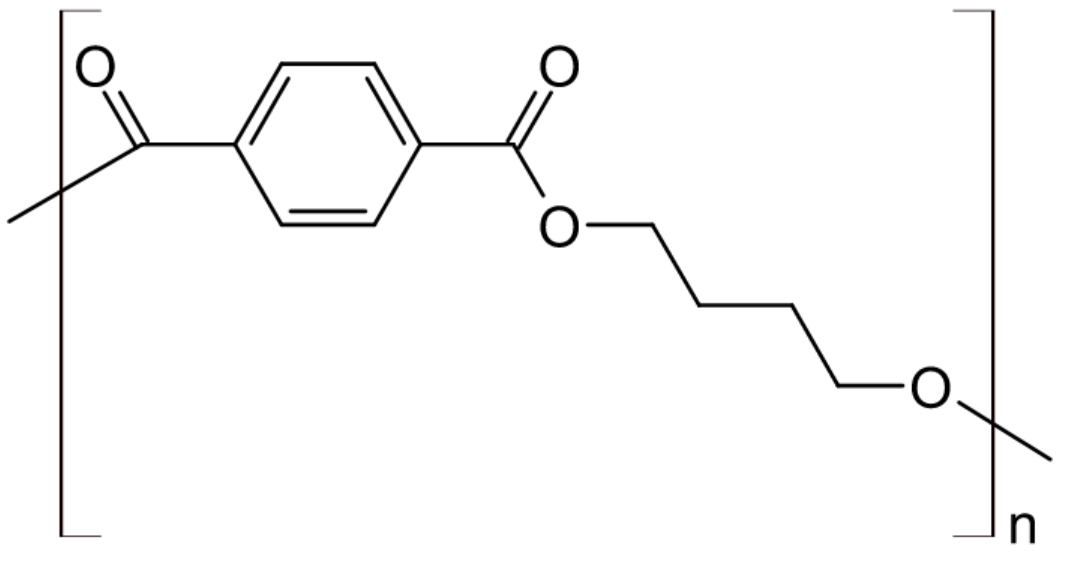
| Crastin®, Celanex®, |
| Polytrimethylene terephthalate (PTT) |
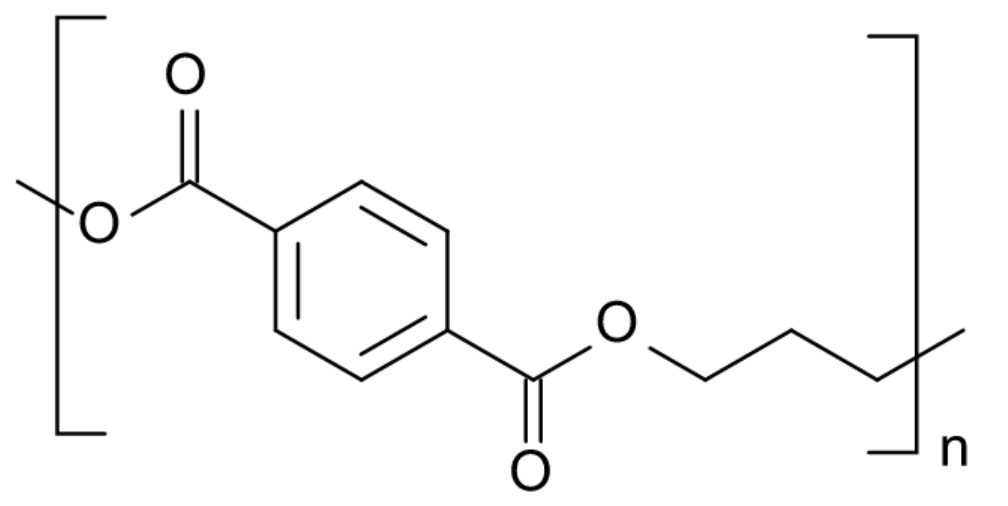
| Sorona® |
| Polyethylene naphthalate (PEN) |
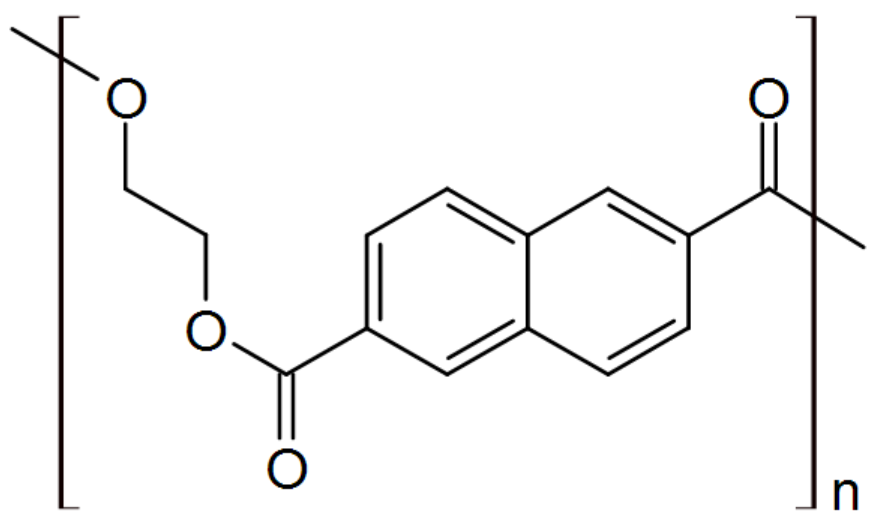
|
Teonex® |
APPLICATIONS
PET is the most widely used polyester. It finds major uses in the textile and packaging industries. PET Fibers (Mylar, Dacron, and Terylene) have excellent crease and wear resistance and low moisture absorption, and cloth made from PET exhibts good wrinkle resistance. As a thermoplastic, PET is mainly used for the production of films (BOPET) and blow-molded bottles for carbonated soft drinks. Other uses of (filled) PET include handles and housings for appliances (cookers, toasters, shower heads, industrial pump housings etc.) In engineering applications, PET is often reinforced with glass fibers or compounded with silicates, graphite and other fillers to improve strength and rigidity. The glass reinforced grades are rated for continuous use temperatures up to 410 - 430 K.
Another high volume polyester is PBT. It has a sligtly lower melting point but greater chain flexibility. PBT crystallizes much faster than PET, and competes with PET in engineering plastics applications. Its maximum use temperature is 390 - 410 K, slightly lower than that of PET. It finds many uses as an engineering thermoplastic, particularly in electrical engineering and in automotive construction. Some examples are plug connectors, relays, keyboards, switches, distribution boxes, and fiber optic cable jackets.
Polytrimethylene terephthalate (PTT) finds uses in the textile industry. It has excellent durability and stain resistance and can be blended with other natural and man-made fibers to enhance the performance of the textile. It finds major uses in variety of markets including carpet and automotive flooring.
Another important polyester is polyethylene naphthalate (PEN). Due to its low oxygen permeability, it is particularly well-suited for bottling beverages that are susceptible to oxidation. It withstands heat and harsh chemicals and has excellent hydrolytic resistance. Its performance is between durable polyester and high performance polyimides. It finds uses as industrial fibers, films, and containers. Some examples are flexible printed circuits, labels, laminates, and optical displays. PEN is also used as a high performance fiber. It has a very high modulus and better dimensional stability than other polyester or nylon fibers.
Fully aromatic polyesters are produced on a very small scale. They are rather expensive and find some applications in the aerospace industry, as well as in some other industries as high performance arylate fibers for very demanding applications. Their performance competes with that of aramide fibers. Some applications include cut resistant gloves, chain saw chaps, high pressure inflatables, abrasion-resistant fabrics, and chemically resistant gaskets.
1The global market
of biodegradable plastics is to reach ca. $3.5 billion by 2022, growing at CAGR of approx. 11.5 percent.
Source: Global Information Inc., Market Research Report Global Biodegradable Plastics Market Insights, June 13, 2016
THERMO-PHYSICAL PROPERTIES
- Poly(Bisphenol A isophthalate)
- Poly(Bisphenol A terephthalate)
- Poly(butylene isophthalate)
- Poly(butylene sebacate)
- Poly(butylene succinate)
- Poly(butylene terephthalate)
- Poly(ethylene sebacate)
- Poly(caprolactone)
- Poly(ethylene adipate)
- Poly(ethylene isophthalate)
- Poly(ethylene naphthalate)
- Poly(ethylene phthalate)
- Poly(ethylene terephthalate)
- Polyglycolide
- Poly(hexylene sebacate)
- Polyhydroxybutyrate
- Polylactic acid
- Poly(trimethylene terephthalate)